About Company:
Torrent Pharma, with annual revenues of more than Rs 10,700 crores, is the flagship Company of the Torrent Group, with group revenues of Rs 41,000 crores. It is ranked 5th in the Indian Pharma Market and is among the Top 5 in the therapeutic segments of Cardiovascular (CV), Central Nervous System (CNS), Gastro-intestinal (GI), Vitamins Minerals Nutritionals (VMN) and Cosmo-Dermatology. The Company also has significant presence in diabetology, pain management, gynaecology, oncology and anti-infective segments. Torrent has 8 manufacturing facilities , of which 5 are USFDA approved. With R&D as the backbone for its growth in domestic & overseas market, it has invested significantly in R&D capabilities with state-of-the-art R&D infrastructure employing around 800 scientists. The acquisition of Elder Pharma’s Indian branded business in 2013, Dermaceuticals business of Zyg Pharma in 2015, API plant of Glochem Industries in 2016, Women healthcare brands from Novartis and Unichem’s Indian branded business along with its Sikkim Plant in 2017 strengthened Torrent Pharma’s position in the Indian Pharma market. Torrent Pharma started international acquisitions in 2005 with entry into the German market. Today, the Company has presence in more than 50 countries and is ranked No. 1 among the Indian pharma Companies in Brazil and Germany. Torrent Pharma is committed towards “not just healthcare but lifecare.”
Vacancy Details:
Job Description:
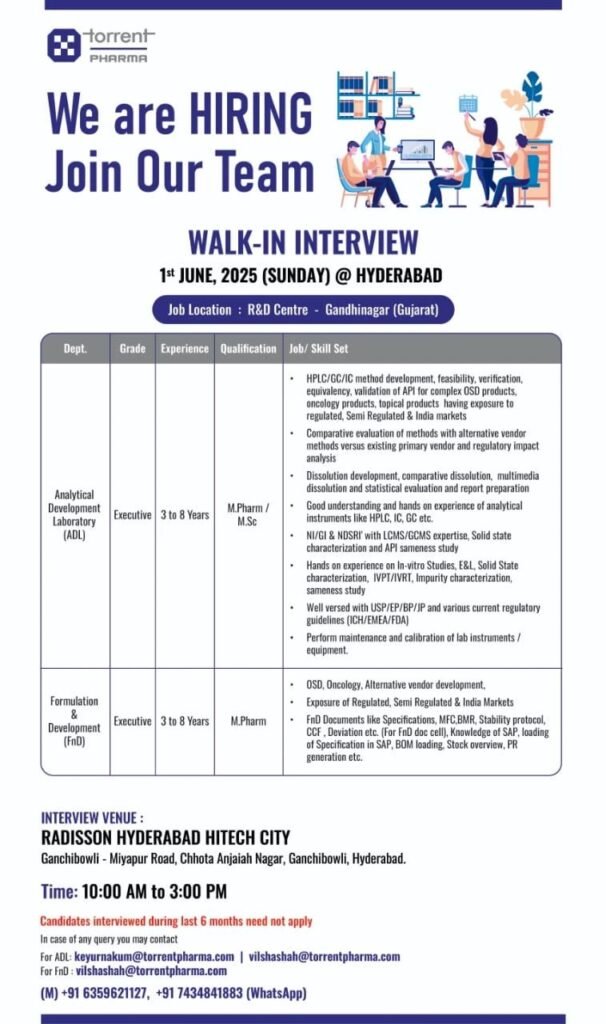
We are HIRING Join Our Team
WALK-IN INTERVIEW
Department: Analytical Development Laboratory (ADL)
Grade: Executive
Experience: 3 to 8 Years
Qualification: M. Pharm/ M. Sc
Job Skill Set:
HPLC/GC/IC method development, feasibility, verification, equivalency, validation of API for complex OSD products. oncology products, topical products having exposure to regulated, Semi Regulated & India markets
Comparative evaluation of methods with alternative vendor methods versus existing primary vendor and regulatory impact analysis
Dissolution development, comparative dissolution, multimedia dissolution and statistical evaluation and report preparation Good understanding and hands on experience of analytical instruments like HPLC, IC, GC etc.
NI/GI & NDSRI’ with LCMS/GCMS expertise, Solid state characterization and API sameness study
Hands on experience on In-vitro Studies, E&L, Solid State characterization, IVPT/IVRT, Impurity characterization, sameness study
Well versed with USP/EP/BP/JP and various current regulatory guidelines (ICH/EMEA/FDA)
Perform maintenance and calibration of lab instruments/ equipment.
Department: Formulation & Development
Grade: Executive
Experience: 3 to 8 Years
Qualification: M. Pharm
Job Skill Set:
OSD, Oncology, Alternative vendor development,
Exposure of Regulated, Semi Regulated & India Markets
FnD Documents like Specifications, MFC,BMR, Stability protocol, CCF, Deviation etc. (For FnD doc cell), Knowledge of SAP, loading of Specification in SAP, BOM loading. Stock overview, PR generation etc.
How to Apply?
INTERVIEW VENUE:
RADISSON HYDERABAD HITECH CITY , Ganchibowli – Miyapur Road, Chhota Anjaiah Nagar, Ganchibowli, Hyderabad.
Date: 1st June 2025(Sunday)@Hyderabad
Time:10:00 AM to 3:00 PM
Job Location: R&D Centre Gandhinagar (Gujarat)
Candidates interviewed during last 6 months need not apply
In case of any query you may contact
For ADL: keyurnakum@torrentpharma.com | vilshashah@torrentpharma.com
For FnD: vilshashah@torrentpharma.com
(M) +91 6359621127, +91 7434841883 (WhatsApp)