Ipca Laboratories Jobs 2025 in Engineering, Production & QA Departments.
Kickstart your career with Ipca Laboratories — a trusted name in the global pharmaceutical industry since 1949!
Ipca Laboratories Limited invites freshers and experienced professionals to attend a Walk-In Interview for Engineering, Production, and Quality Assurance (API) Departments at their Indore facility.
About Ipca Laboratories:
Founded in 1949, Ipca Laboratories Limited is a leading, consumer-driven global pharmaceutical company operating in 120+ countries across six continents.
With over 350 formulations and 80 active pharmaceutical ingredients (APIs), Ipca is one of the world’s top manufacturers and exporters of APIs, approved by regulatory bodies such as UK-MHRA, EDQM-Europe, and WHO-Geneva.
Key Highlights:
- Leadership in DMARDs (Disease Modifying Anti-Rheumatic Drugs) for Rheumatoid Arthritis
- Leading brands in Pain Management, Rheumatology, Antimalarial, and Hair Care segments
- 15 API & 11 formulation manufacturing facilities worldwide
- Recognized globally for excellence in GMP-compliant manufacturing
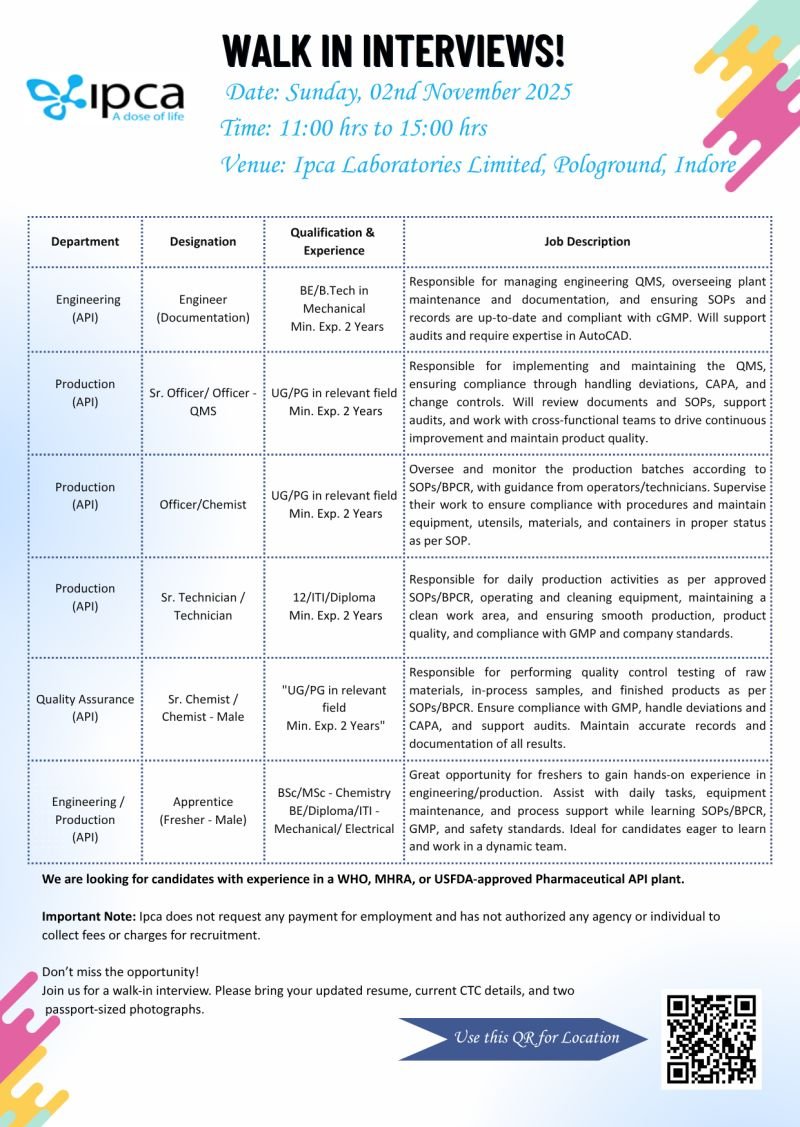
Walk-In Interview Details:
Date: Sunday, 2nd November 2025
Time: 11:00 AM – 3:00 PM
Venue: Ipca Laboratories Limited, Pologround, Indore
Vacancy Details:
| Department | Designation | Qualification & Experience | Job Description |
| Engineering (API) | Engineer (Documentation) | BE/B.Tech in Mechanical, Min. 2 Years Exp. | Manage engineering QMS, plant maintenance, and ensure cGMP compliance using AutoCAD. |
| Production (API) | Sr. Officer / Officer – QMS | UG/PG in relevant field, Min. 2 Years Exp. | Maintain QMS, handle deviations, CAPA, and audits to ensure product quality. |
| Production (API) | Officer / Chemist | UG/PG in relevant field, Min. 2 Years Exp. | Supervise production batches, monitor SOPs/BPCR compliance, and maintain process quality. |
| Production (API) | Sr. Technician / Technician | 12th / ITI / Diploma, Min. 2 Years Exp. | Perform daily production, operate and clean equipment, ensure GMP compliance. |
| Quality Assurance (API) | Sr. Chemist / Chemist – Male | UG/PG in relevant field, Min. 2 Years Exp. | Conduct quality control of raw materials, in-process, and finished goods per SOPs. |
| Engineering / Production (API) | Apprentice (Fresher – Male) | B.Sc / M.Sc – Chemistry / BE / Diploma / ITI – Mech / Electrical | Hands-on training in production & maintenance, learning GMP, safety standards, and process optimization. |
Who Can Apply:
- Candidates with experience in WHO, MHRA, or USFDA-approved pharmaceutical API plants
- Freshers seeking pharma apprenticeship opportunities
- Engineering, Chemistry, or Life Science graduates passionate about pharma production and QA
What to Bring:
- Updated Resume
- Current CTC details
- Two passport-size photographs
Note: Ipca does not charge any fees for recruitment. Beware of fraudulent offers.
Why Choose Ipca Laboratories?
- Work with globally recognized pharmaceutical experts
- Opportunities for continuous learning and professional growth
- Exposure to advanced GMP systems and international standards
- Collaborative and safety-focused workplace