About Company:
A consumer-led global pharmaceutical company, creating healthy doses of life since 1949. When you operate in an industry like pharmaceuticals, your work goes way beyond creating ‘products for customers’. It is different from any other domain – there lies a higher sense of responsibility and a need for utmost integrity in everything you do. As you serve millions of lives, high quality standards become a pre-requisite, and safety of your people and consumers always comes first. All this, while ensuring that each life you touch is treated with respect and dignity. For more than 60 years, Ipca has been a crucial healthcare partner in over 120 countries across the 6 continents. We are a fully-integrated pharmaceutical company that manufactures over 350 formulations and 80 APIs for various therapeutic segments. Today, we are one of the world’s largest manufacturers and suppliers of over a dozen APIs. These are produced from scratch at fully-automated manufacturing facilities, approved by the world’s most discerning drug regulatory authorities like UK-MHRA, EDQM-Europe, and WHO-Geneva, among others. – One of the largest suppliers of these APIs worldwide with manufacturing leadership in over 12 APIs globally – 15 APIs & 11 Formulations manufacturing facility across the globe Leader in DMARDs (Disease Modifying Anti-Rheumatic Drugs) treatment for Rheumatoid Arthritis – Leading brands in Pain, Rheumatology, Antimalarials and Hair care therapy – 4 formulations rank amongst the top 300 brands of IPM as per IQVIA
Vacancy Details:
Job Description:
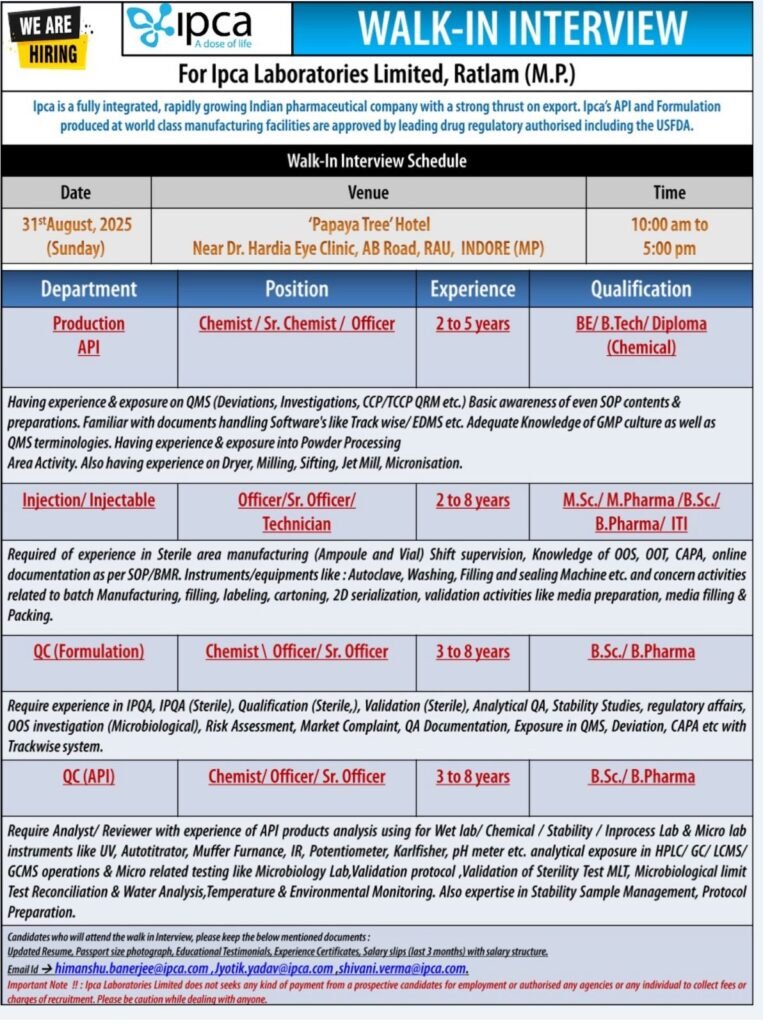
WALK-IN INTERVIEW
For Ipca Laboratories Limited, Ratlam (M.P.)
Ipca is a fully integrated, rapidly growing Indian pharmaceutical company with a strong thrust on export. Ipca’s API and Formulation produced at world class manufacturing facilities are approved by leading drug regulatory authorised including the USFDA.
1.Department: Production ΑΡΙ
Position: Chemist/Sr. Chemist/ Officer
Experience: 2 to 5 years
Qualification: BE/B.Tech/Diploma (Chemical)
Having experience & exposure on QMS (Deviations, Investigations, CCP/TCCP QRM etc.) Basic awareness of even SOP contents & preparations. Familiar with documents handling Software’s like Track wise/EDMS etc. Adequate Knowledge of GMP culture as well as QMS terminologies. Having experience & exposure into Powder Processing
Area Activity. Also having experience on Dryer, Milling, Sifting, Jet Mill, Micronisation.
2.Department: Injection/Injectable
Position: Officer/Sr. Officer/ Technician
Experience: 2 to 8 years
Qualification: M.Sc./ M.Pharma/B.Sc./ B.Pharma/ ITI
Required of experience in Sterile area manufacturing (Ampoule and Vial) Shift supervision, Knowledge of OOS, OOT, CAPA, online documentation as per SOP/BMR. Instruments/equipments like: Autoclave, Washing, Filling and sealing Machine etc. and concern activities related to batch Manufacturing, filling, labeling, cartoning, 2D serialization, validation activities like media preparation, media filling & Packing.
3.Department: QC (Formulation)
Position: Chemist Officer/ Sr. Officer
Experience: 3 to 8 years
Qualification: B.Sc./B.Pharma
Require experience in IPQA, IPQA (Sterile), Qualification (Sterile,), Validation (Sterile), Analytical QA, Stability Studies, regulatory affairs, OOS investigation (Microbiological), Risk Assessment, Market Complaint, QA Documentation, Exposure in QMS, Deviation, CAPA etc with Trackwise system.
4.Department: QC (API)
Position: Chemist/Officer/Sr. Officer
Experience: 3 to 8 years
Qualification: B.Sc./B.Pharma
Require Analyst/Reviewer with experience of API products analysis using for Wet lab/ Chemical/Stability/Inprocess Lab & Micro lab instruments like UV, Autotitrator, Muffer Furnance, IR, Potentiometer, Karlfisher, pH meter etc. analytical exposure in HPLC/GC/ LCMS/ GCMS operations & Micro related testing like Microbiology Lab,Validation protocol,Validation of Sterility Test MLT, Microbiological limit Test Reconciliation & Water Analysis, Temperature & Environmental Monitoring. Also expertise in Stability Sample Management, Protocol Preparation.
Candidates who will attend the walk in Interview, please keep the below mentioned documents:
Updated Resume, Passport size photograph, Educational Testimonials, Experience Certificates, Salary slips (last 3 months) with salary structure.
How to Apply?
Walk-In Interview Schedule
Date: 31st August, 2025 (Sunday)
Time: 10:00 am to 5:00 pm
Venue: ‘Papaya Tree’ Hotel Near Dr. Hardia Eye Clinic, AB Road, RAU, INDORE (MP)
Email Id: himanshu.banerjee@ipca.com,Jyotik.yadav@ipca.com,shivani.verma@ipca.com.
Important Note !!: Ipca Laboratories Limited does not seeks any kind of payment from a prospective candidates for employment or authorised any agencies or any individual to collect fees or charges of recruitment. Please be caution while dealing with anyone.