About Company:
Welcome to the transformative world of GUFIC Biosciences, where a legacy of innovation, spanning over five decades, converges with an unwavering commitment to global well-being. Our Noble Mission: At the heart of our journey lies a mission that transcends boundaries – to lead in specialized medicinal segments while making high-quality products accessible globally. We excel in critical care medicines, premium fertility and parenthood solutions, potent nutraceutical formulations, and advanced medications for orthopaedic and gynaecology treatments. Additionally, we are venturing into delivering cutting-edge aesthetic and derma solutions. Gufic was the first to start Lyophilization technology and has now progressed with our benchmark setting fully automated robotic armed Lyophilizers. The manufacturing facility is accredited by EU-GMP, ANVISA- Brazil, TGA- Australia, HC- Canada, SAHPRA- South Africa and many more regulatory accreditations globally. The exclusive manufacturing facility for API is focused on developing non-infringing, novel, cost-effective and scalable chemical process for APIs with presence in more than 25 countries globally. Gufic Biosciences isn’t just a company; it’s an inspiration, a symbol of transformative healthcare. Join us in redefining pharmaceutical excellence and shaping a brighter, healthier future for all. Welcome to GUFIC, where every step is a stride towards hope and well-being.
Vacancy Details:
Job Description:
Dear All
We, GUFIC are hiring passionate and talented individuals to join us snd become a part of growing company
Dreaming of your next big career move ???
Then catch this opportunity as this is your moment to come for walk in interview dated 2nd August 25 at Gufic Biosciences limited navsari Gujarat plant. Don’t miss this chance to be part of a workplace that value three “P”
Progress, People & Performance
GUFIC BIOSCIENCES LIMITED
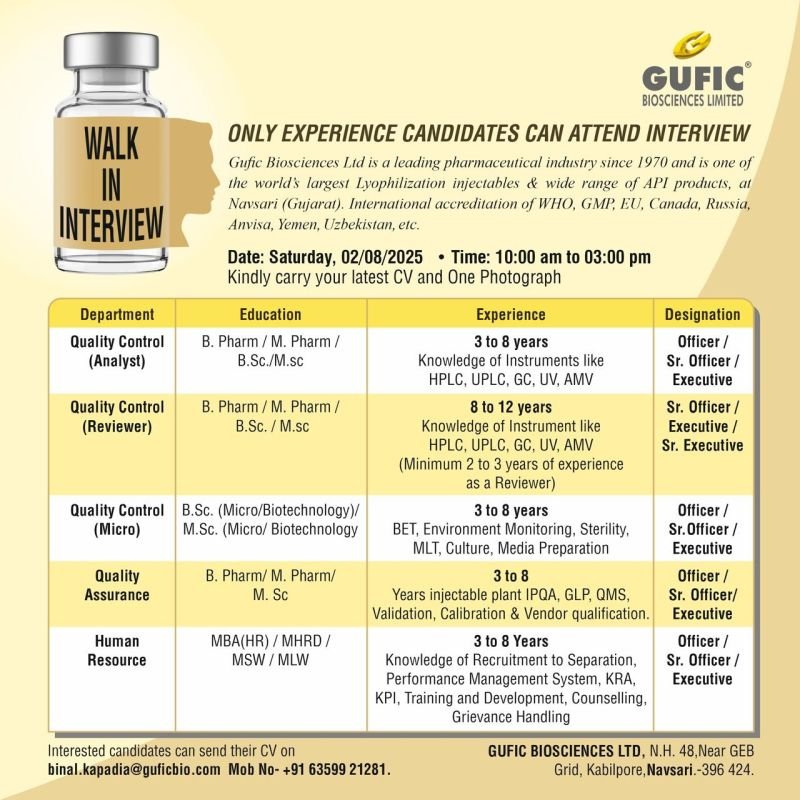
ONLY EXPERIENCE CANDIDATES CAN ATTEND INTERVIEW
Gufic Biosciences Ltd is a leading pharmaceutical industry since 1970 and is one of the world’s largest Lyophilization injectables & wide range of API products, at Navsari (Gujarat). International accreditation of WHO, GMP, EU, Canada, Russia, Anvisa, Yemen, Uzbekistan, etc.
1.Department: Quality Control (Analyst)
Education: B. Pharma/ M.Pharma/ B.Sc./M. Sc
Experience: 3 to 8 years
Knowledge of Instruments like HPLC, UPLC, GC, UV, AMV
Designation: Officer/ Sr. Officer/ Executive
2.Department: Quality Control (Reviewer)
Education: B. Pharma/ M. Pharma/ B.Sc./M.Sc
Experience: 8 to 12 years Knowledge of Instrument like HPLC, UPLC, GC, UV, AMV (Minimum 2 to 3 years of experience as a Reviewer)
Designation: Sr. Officer/ Executive/ Sr. Executive
3.Department: Quality Control (Micro)
Education: B.Sc. (Micro/Biotechnology)/ M.Sc. (Micro/ Biotechnology
Experience: 3 to 8 years
BET, Environment Monitoring, Sterility, MLT, Culture, Media Preparation
Designation: Officer/ Sr. Officer/ Executive
4.Department: Quality Assurance
Qualification: B. Pharma/ M. Pharma/ M. Sc
Experience: 3 to 8
Years injectable plant IPQA, GLP, QMS, Validation, Calibration & Vendor qualification.
Designation: Officer/ Sr. Officer/ Executive
5.Department: Human Resource
Qualification: MBA(HR)/MHRD/ MSW/MLW
Experience: 3 to 8 Years
Knowledge of Recruitment to Separation, Performance Management System, KRA, KPI, Training and Development, Counselling, Grievance Handling
Designation: Officer/ Sr. Officer/ Executive
How to Apply?
Walk-in Details:
Date: Saturday, 02/08/2025
Time: 10:00 am to 03:00 pm
Interested candidates can send their CV on
binal.kapadia@guficbio.com
Mob No- +91 63599 21281.
GUFIC BIOSCIENCES LTD, N.H. 48, Near GEB Grid, Kabilpore, Navsari.-396 424.
Kindly carry your latest CV and One Photograph