About Company:
At Endo, our far-reaching vision is simple: to help everyone we serve live their best life. As a specialty pharmaceutical company, we’re motivated by a strong sense of purpose to find better ways to meet unique medical needs.
Our global team of passionate employees understands the importance of their work. We’re dedicated to supporting one another as we connect with communities and foster partnerships that elevate quality-of-life and bring the best treatments forward.
Our uncompromising commitment results in the delivery of life-enhancing therapies. From intelligent product selection to commercialization, we strive to make a meaningful, tangible impact to help everyone live their best life.
Endo has global headquarters in Malvern, Pennsylvania.
Community Guidelines:
- Be respectful. Everyone who visits our page should feel comfortable and respected.
- If we see a comment that violates anything in the following list, it may be removed.
- Comments that use profanity; personally attack or bully another individual; or are off-topic, misleading, factually inaccurate, political, spam, defamatory, discriminatory or promotional.
- Comments that are excessively repetitive and/or disruptive to the community.
- Comments that promote illegal activity, use copyrights or trademarks or are related to an ongoing legal matter.
- Comments that appear to be medical advice.
We reserve the right to remove a reply for any reason at any time.
- Adverse Event Reporting: If we see a post about an adverse event, an Endo representative will need to contact you to find out more information to comply with regulatory guidelines. If you experience a side effect while using an Endo product, please consult your physician or pharmacist immediately. You may also report to the FDA at fda.gov/medwatch or 800-FDA-1088.
Replies from other users do not necessarily reflect the views of Endo. We do not endorse content added by other users.
Vacancy Details:
Job description:
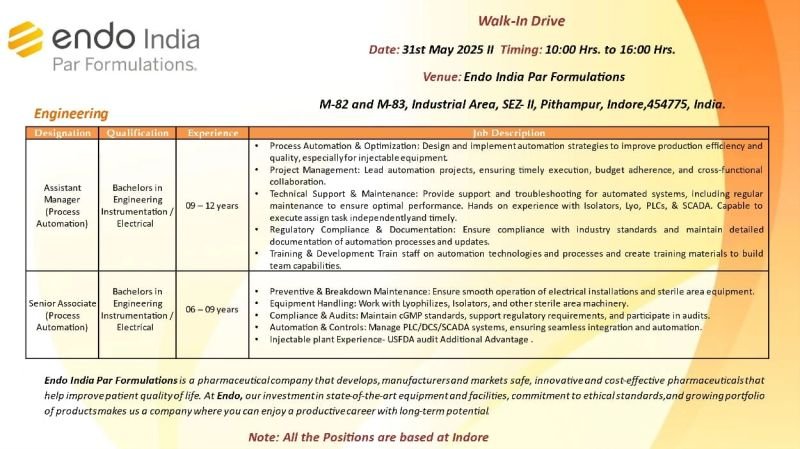
Walk-In Drive
1.Microbiology
Designation: Analyst
Qualification: M.Sc. Microbiology
Experience: 02-06 Years
Job Description:
Environmental Monitoring:
To execute microbial environmental monitoring program in manufacturing environments using passive air sampling, active air sampling and surface monitoring to evaluate the cleanliness levels Knowledge of aseptic behavior.
Preferences:
Exposure in digital platforms like, LIMS and MODA, KTA, Exposure in microbiological method development and validation Exposure in Isolator technology, Aseptic process simulations, USFDA inspections.
Designation: Sr. Analyst
Qualification: M.Sc. Microbiology
Experience: 07-10 Years
Laboratory Operations:
Review and approve of laboratory records, lab generated data to ensure compliance with standard operating procedures and regulatory requirements Responsible for initiating QMS elements like Deviation, change control and CAPA items. Preparation/ review of SOPs, Testing results, calibration records, validation protocols and reports, Microbiological study reports, Vitek 2 review and logbooks.
Preferences:
Exposure in track wise, Electronic data platforms, Trouble shooting, skills on investigations, technical write-up.
Endo India Par Formulations is a pharmaceutical company that develops, manufacturersand markets safe, innovative and cost-effective pharmaceuticals that help improve patient quality of life. At Endo, our investment in state-of-the-art equipment and facilities, commitment to ethical standards, and growing portfolio of products makes us a company where you can enjoy a productive career with long-term potential
2,Engineering
Designation: Assistant Manager(Process Automation)
Qualification: Bachelors in Engineering Instrumentation/ Electrical
Experience: 09-12 years
Job Description
Process Automation & Optimization: Design and implement automation strategies to improve production efficiency and quality, especially for injectable equipment
Project Management: Lead automation projects, ensuring timely execution, budget adherence, and cross-functional collaboration.
Technical Support & Maintenance: Provide support and troubleshooting for automated systems, including regular maintenance to ensure optimal performance. Hands on experience with Isolators, Lyo, PLCs, & SCADA. Capable to execute assign task independently and timely.
Regulatory Compliance & Documentation: Ensure compliance with industry standards and maintain detailed documentation of automation processes and updates.
Training & Development Train staff on automation technologies and processes and create training materials to build team capabilities.
Designation: Senior Associate (Process Automation)
Qualification: Bachelors in Engineering Instrumentation/Electrical
Experience: 06-09 years
Job Description:
Preventive & Breakdown Maintenance: Ensure smooth operation of electrical installations and sterile area equipment.
Equipment Handling: Work with Lyophilizes, Isolators, and other sterile area machinery.
Compliance & Audits: Maintain cGMP standards, support regulatory requirements, and participate in audits.
Automation & Controls: Manage PLC/DCS/SCADA systems, ensuring seamless integration and automation.
Injectable plant Experience- USFDA audit Additional Advantage.
Endo India Par Formulations is a pharmaceutical company that develops, manufacturers and markets safe, innovative and cost-effective pharmaceuticals that help improve patient quality of life. At Endo, our investment in state-of-the-art equipment and facilities, commitment to ethical standards, and growing portfolio of products makes us a company where you can enjoy a productive career with long-term potential
3.Engineering
Designation: Assistant Manager(Process maintenance)
Qualification: Bachelors in Engineering (Mechanical/Electrical)
Experience: 09-12 years
Job Description
Oversee mechanical and electrical maintenance activities, especially for PFS lines and isolators. Plan and implement preventive maintenance schedules to ensure equipment reliability. Troubleshoot mechanical/electrical issues to minimize downtime.
Ensure compliance with safety regulations and industry standards; CMMS knowledge essentiall.
Lead, train, and support a team of technicians with effective communication.
Manage critical spare parts inventory.
Maintain accurate documentation of all maintenance tasks.
Support execution of equipment upgrades and maintenance projects.
Designation: Sr Associate (Water Systems)
Qualification: Bachelors in Engineering Mechanical/ Electrical
Experience: 06-09 years
Job Description:
Regulatory Compliance: Ensure adherence to cGMP, FDA, and WHO guidelines for water system validation.
Preventive & Breakdown Maintenance: Conduct routine checks and troubleshooting of Reverse Osmosis (RO), Multi Effect Distillation (MED), and Vapor Compression Distillation (VCD) units.
Automation & Monitoring: Manage SCADA/DCS systems for real-time monitoring and control.
Microbial & Chemical Testing: Collaborate with Quality teams to ensure water quality meets pharmaceutical standards.
Documentation & Audits: Maintain records for water system validation, calibration, and regulatory audits. Injectable plant Experience-USFDA audit Additional Advantage.
Endo India Par Formulations is a pharmaceutical company that develops, manufacturers and markets safe, innovative and cost-effective pharmaceuticals that help improve patient quality of life. At Endo, our investment in state-of-the-art equipment and facilities, commitment to ethical standards, and growing portfolio of products makes us a company where you can enjoy a productive career with long-term potential
Note: All the Positions are based at Indore
How to Apply?
Date: 31st May 2025
Timing: 10:00 Hrs. to 16:00 Hrs.
Venue: Endo India Par Formulations M-82 and M-83, Industrial Area, SEZ- II, Pithampur, Indore,454775, India.