About Company:
Dishman is a global, dynamic group of companies offering a continuum of services to the pharmaceutical industry. We are a global outsourcing partner for pharmaceutical companies, offering a portfolio of products and development, scale-up and manufacturing services. The Dishman Group continually invests in the pharmaceutical industry, ensuring Dishman’s businesses can provide pharmaceutical customers with high-value, high-quality products and services today and in the future. Our focus is to add value to the global pharmaceutical industry by serving as a reliable partner. Our business is successful only when our customers are successful.
Vacancy Details:
Job description:
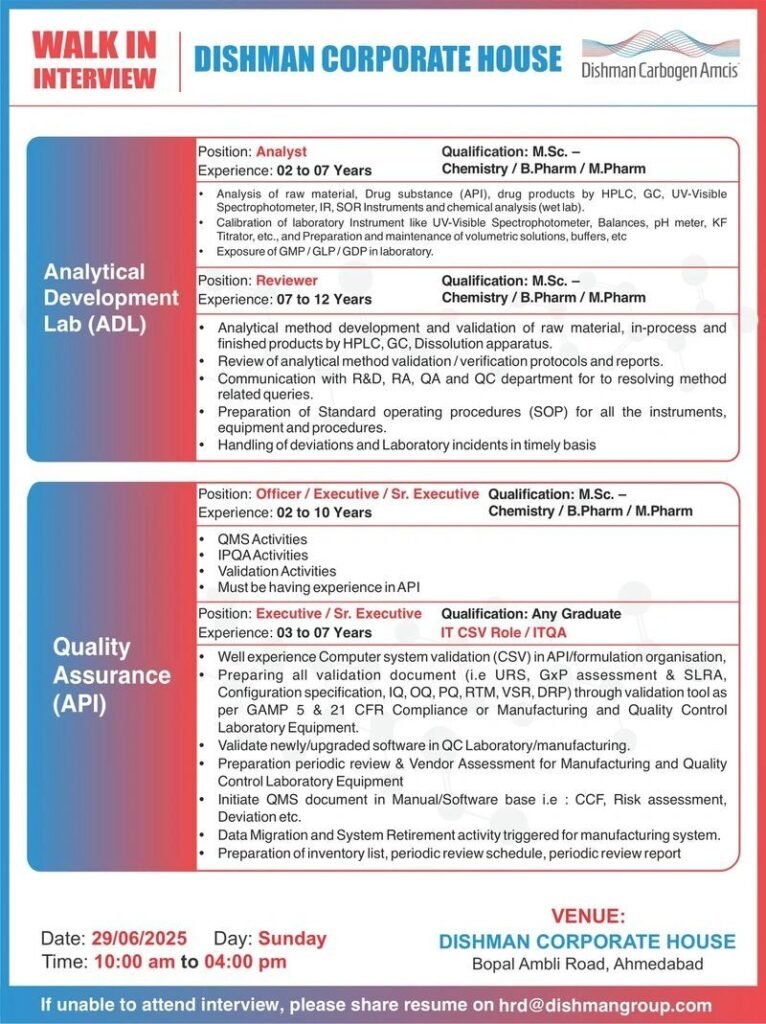
WALK IN INTERVIEW
DISHMAN CORPORATE HOUSE
Dishman Carbogen Amcis
1.Department: Analytical development Lab(ADL)
Position: Analyst
Experience: 02 to 07 Years
Qualification: M.Sc. – Chemistry /B. Pharm / M. Pharm
1.Analysis of raw material, Drug substance (API), drug products by HPLC, GC, UV-Visible Spectrophotometer, IR, SOR Instruments and chemical analysis (wet lab).
2.Calibration of laboratory Instrument like UV-Visible Spectrophotometer, Balances, pH meter, KF Titrator, etc., and Preparation and maintenance of volumetric solutions, buffers, etc
3. Exposure of GMP/GLP/GDP in laboratory.
Position: Reviewer
Experience: 07 to 12 Years
Qualification: M.Sc. – Chemistry /B. Pharm/ M. Pharm
1.Analytical method development and validation of raw material, in-process and finished products by HPLC, GC, Dissolution apparatus.
2.Review of analytical method validation/verification protocols and reports.
3.Communication with R&D, RA, QA and QC department for to resolving method related queries.
4.Preparation of Standard operating procedures (SOP) for all the instruments, equipment and procedures.
5.Handling of deviations and Laboratory incidents in timely basis
2.Department: Quality Assurance (API)
Position: Officer/Executive/Sr. Executive
Qualification: M.Sc. – Chemistry /B. Pharm / M. Pharm
Experience: 02 to 10 Years
1.QMS Activities
2.IPQA Activities
3.Validation Activities
4.Must be having experience in API
Position: Executive/Sr. Executive
Experience: 03 to 07 Years
Qualification: Any Graduate
IT CSV Role/ITQA
1.Well experience Computer system validation (CSV) in API/formulation organisation,
2.Preparing all validation document (i.e URS, GxP assessment & SLRA, Configuration specification, IQ, OQ, PQ, RTM, VSR, DRP) through validation tool as per GAMP 5 & 21 CFR Compliance or Manufacturing and Quality Control Laboratory Equipment.
3.Validate newly/upgraded software in QC Laboratory/manufacturing.
4.Preparation periodic review & Vendor Assessment for Manufacturing and Quality Control Laboratory Equipment
5.Initiate QMS document in Manual/Software base i.e: CCF, Risk assessment, Deviation etc.
6.Data Migration and System Retirement activity triggered for manufacturing system.
7.Preparation of inventory list, periodic review schedule, periodic review report
3.Department: Engineering
Position: Officer to Sr. Executive
Experience: 02 to 07 Years
Qualification: BE / B.Tech. – Instrumentation & Control
Maintenance and Projects in API division
Candidate having Good Knowledge of CSV Validation as per GMP-5 Green Field and brown field project knowledge
Knowledge of instrumentation calibration like, TT, PT, FT, RTD operation and maintenance of all pharmaceutical control instrumentation, Programming and troubleshooting.
Position: Officer to Sr. Executive
Experience: 02 to 07 Years
Qualification: BE/B.Tech. – Mechanical Maintenance & Utility
1.Having good knowledge of operation and maintenance of Purified water System, HVAC and utility equipment like Chiller, Air Compressor, hot water plant.
2.Handle QMS documents like change control, Deviation, Qualification Protocols, SOPs etc
3.Maintenance in reactor, centrifuge, fluid bed dryer, rotary vacuum dryer, agitated nutch filter, centrifugal pump, sparkler filter, etc.
4. Department: Research & Development
Position: Research Associate
Experience: 02 to 07 Years
Qualification: M.Sc. – Organic Chemistry
Candidate should be experienced in process development in R&D in API synthesis Technology transfer, scale-up and troubleshooting of Lab developed process at Pilot and Commercial level.
To carry experimental work for development/improvement of the products assign
Position: Analytical Associate
Experience: 02 to 07 Years
Qualification: M.Sc. – Analytical Chemistry
1.Well conversant with operating, handling and troubleshooting in HPLC, GC To maintain analytical records as per IMS.
2.Development Co-ordinate with ADL dept./Q.C dept. for method development activity.
3.Development or improvement of methods for the assigned project
Position: Research Scientist
Experience: 10 to 15 Years
Qualification: M.Sc./Ph. D – Organic Chemistry
Development / improvement of the assigned products and overall review of development activities.
To finalize route based on literature or feasibility of process or availability of raw materials
Technology transfer, scale-up and troubleshooting of Lab developed process at Pilot and Commercial level
How to Apply?
VENUE:
Date: 29/06/2025
Day: Sunday
Time: 10:00 am to 04:00 pm
DISHMAN CORPORATE HOUSE Bopal Ambli Road, Ahmedabad
If unable to attend interview, please share resume on hrd@dishmangroup.com