About Company:
Ajanta Pharma Limited (APL) is a pharmaceutical company headquartered in Mumbai, India. It has strong presence in Branded Generic business in India & Emerging markets; and Generic business in USA. In India, company operates in selected therapeutic areas of Cardiology, Dermatology, Ophthalmology and Pain management. Its brands hold leadership positions in sub-therapeutic areas they are present in. In Emerging Markets, company has presence in Africa, South East Asia, West Asia, and CIS on broader therapeutic segments such as antimalarial, gastro, antibiotics, cardiology, dermatology, pain management, etc. As on 30th Sept 2024, Ajanta has 46 ANDA approvals which are commercialised. It holds 2 tentative approvals and 22 ANDAs are awaiting US FDA approval. Company plans to file 8-12 ANDAs during the current financial year. Company has state-of-the-art research facilities for formulation and API development located at Mumbai, India. R&D capabilities are evident from number of 1st to market products launched by the company providing patients most needed compliance and convenience. A dedicated and focused team of over 800 scientists work for R&D, which is growing continuously. Ajanta has 6 formulations manufacturing facilities located in India. Besides that, it also has an API manufacturing facility located at Waluj, India. Ajanta’s flagship formulation facilities at Paithan (Maharashtra, India) and Dahej (Gujarat, India) have been approved by US FDA. Ajanta continuously invests in enhancing the existing manufacturing facilities to meet current cGMP requirements and also construct new facilities to meet company’s growth requirements. Please visit https://ajantapharma.com/ for more information.
Vacancy Details:
Job Description:
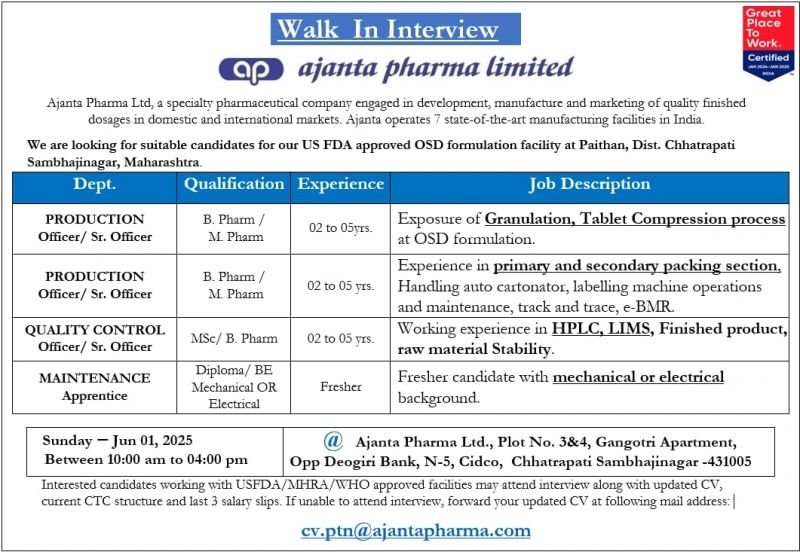
Walk In Interview
ajanta pharma limited
Ajanta Pharma Ltd, a specialty pharmaceutical company engaged in development, manufacture and marketing of quality finished dosages in domestic and international markets. Ajanta operates 7 state-of-the-art manufacturing facilities in India.
We are looking for suitable candidates for our US FDA approved OSD formulation facility at Paithan, Dist. Chhatrapati Sambhajinagar, Maharashtra.
1.Department: PRODUCTION Officer/ Sr. Officer
Qualification: B. Pharm/M. Pharm
Experience: 2-5 years
Job description: Exposure of Granulation, Tablet Compression process at OSD formulation.
2.PRODUCTION Officer/ Sr. Officer
Qualification: B. Pharm/M. Pharm
Experience: 2-5 years
Job Description: Experience in primary and secondary packing section, 02 to 05 yrs. Handling auto cartonator, labelling machine operations and maintenance, track and trace, e-BMR.
3.Department: QUALITY CONTROL Officer/ Sr. Officer
Qualification: M. Sc/B. Pharm
Experience: Freshers
Job Description: Working experience in HPLC, LIMS, Finished product, raw material Stability.
4.Department: MAINTENANCE Apprentice
Qualification : Diploma/BE Mechanical or Electrical
Experience: Fresher
Job Description: Fresher candidate with mechanical or electrical background.
How to Apply?
Location: @Ajanta Pharma Ltd., Plot No. 3&4, Gangotri Apartment, Opp Deogiri Bank, N-5, Cidco, Chhatrapati Sambhajinagar -431005
Date & Time: 01 June 2025(Sunday) Between 10:00 am to 04:00 pm
Interested candidates working with USFDA/MHRA/WHO approved facilities may attend interview along with updated CV, current CTC structure and last 3 salary slips.
If unable to attend interview, forward your updated CV at following mail address: cv.ptn@ajantapharma.com