About Company:
Ami Lifesciences was founded with the goal of changing the way in which quality medicines are delivered to people in need. We operate on the principle by extending our focus and services to maximize our impact on the global pharmaceutical industry. We have spent last two decades in building up our scientific and technological capabilities, research, and manufacturing competencies. Along the way we have earned a reputation for being innovative, inventive and resourceful. Our vision is to be a leading pharmaceutical company in India and become a significant global player by providing high quality and affordable API’s. We at Ami Lifesciences are committed to the pursuit of excellence through world-class products, innovative processes and empowered employees to provide the highest level of satisfaction to its customers.
Vacancy Details:
Job Description:
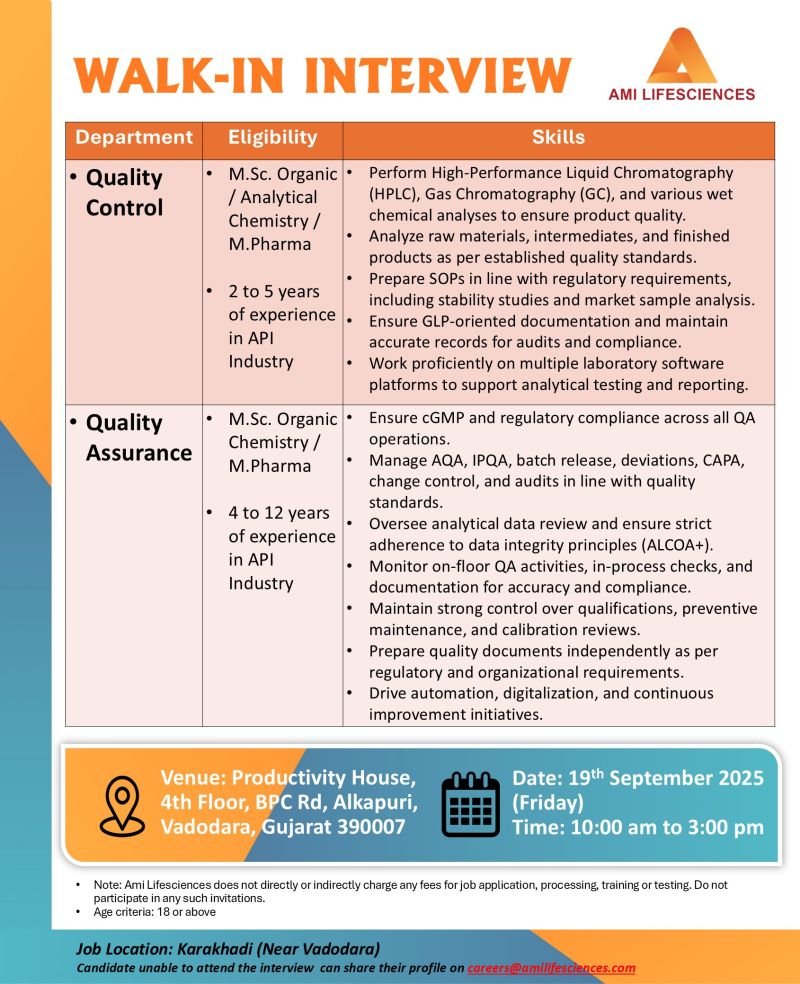
WALK-IN INTERVIEW
1.Department: Quality Control
Eligibility: M.Sc. Organic/ Analytical Chemistry/M.Pharma
Experience: 2 to 5 years of experience. in API Industry
Skills:
1.Perform High-Performance Liquid Chromatography (HPLC), Gas Chromatography (GC), and various wet chemical analyses to ensure product quality.
2.Analyze raw materials, intermediates, and finished products as per established quality standards.
3.Prepare SOPs in line with regulatory requirements, including stability studies and market sample analysis.
4.Ensure GLP-oriented documentation and maintain accurate records for audits and compliance.
5.Work proficiently on multiple laboratory software platforms to support analytical testing and reporting.
2.Department: Quality Assurance
Eligibility: M.Sc. Organic Chemistry/ M.Pharma
Experience: 4 to 12 years of experience in API Industry
Skills:
1.Ensure cGMP and regulatory compliance across all QA operations.
2.Manage AQA, IPQA, batch release, deviations, CAPA, change control, and audits in line with quality standards.
3.Oversee analytical data review and ensure strict adherence to data integrity principles (ALCOA+).
4.Monitor on-floor QA activities, in-process checks, and documentation for accuracy and compliance.
5.Maintain strong control over qualifications, preventive maintenance, and calibration reviews.
6.Prepare quality documents independently as per regulatory and organizational requirements.
7.Drive automation, digitalization, and continuous improvement initiatives.
How to Apply?
Walk-in Details:
Date: 19th September 2025 (Friday)
Time: 10:00 am to 3:00 pm
Venue: Productivity House, 4th Floor, BPC Rd, Alkapuri, Vadodara, Gujarat 390007
Note: Ami Lifesciences does not directly or indirectly charge any fees for job application, processing, training or testing. Do not participate in any such invitations.
Age criteria: 18 or above
Job Location: Karakhadi (Near Vadodara)
Candidate unable to attend the interview can share their profile on careers@amilifesciences.com