About Company:
Ciro Pharma Private Limited is a pharmaceutical formulation company manufacturing anti cancer oncology drugs for regulatory markets. We are set to manufacture all dosage forms tablets, capsules, injections and lyophilized products. The company is into direct marketing of it a products world wide. The Unit 1 of Ciro is specialized Soft Gel capsules manufacturing unit. It’s state of the art equipment and drying rooms give the best output of Soft Gel capsules.
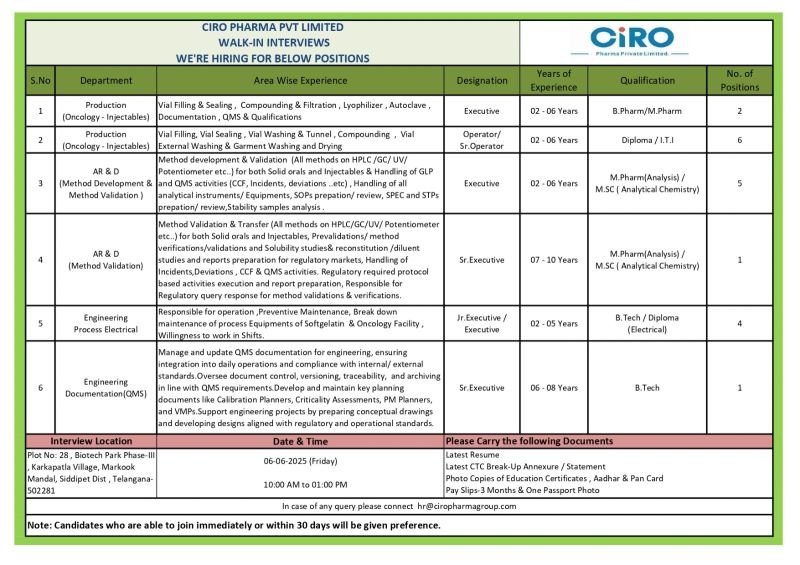
Vacancy Details:
Job Description:
1.Department: Production (Oncology – Injectables)
Area Wise Experience: Vial Filling & Sealing, Compounding & Filtration, Lyophilizer, Autoclave, Documentation, QMS & Qualifications
Designation: Executive
Experience: 2-6 years
Qualification: B. Pharm/M. Pharm
No of Positions: 2
2.Department: Production (Oncology – Injectables)
Area Wise Experience: Vial Filling, Vial Sealing, Vial Washing & Tunnel, Compounding, Vial External Washing & Garment Washing and Drying
Designation: Operator/Sr. operator
Experience: 2-6 years
Qualification: Diploma/ITI
No Of Positions: 6
3.Department: AR & D(Method Development & Method Validation)
Area Wise Experience:
Method development & Validation (All methods on HPLC/GC/UV/ Potentiometer etc..) for both Solid orals and Injectables & Handling of GLP and QMS activities (CCF, Incidents, deviations..etc), Handling of all analytical instruments/ Equipments, SOPs prepation/review, SPEC and STPs prepation/ review, Stability samples analysis.
Designation: Executive
Experience: 2-6 years
Qualification: M. Pharma(Analysis)/M. Sc(Analytical Chemistry)
No of Positions: 5
4.Department: AR & D (Method Validation)
Area Wise Experience: Method Validation & Transfer (All methods on HPLC/GC/UV/Potentiometer etc..) for both Solid orals and Injectables, Pre validations/method verifications/validations and Solubility studies& reconstitution /diluent studies and reports preparation for regulatory markets, Handling of Incidents, Deviations, CCF & QMS activities. Regulatory required protocol based activities execution and report preparation, Responsible for Regulatory query response for method validations & verifications
Designation: Sr. Executive
Experience: 7-10 years
Qualification: M. Pharm Analysis)/ M.SC (Analytical Chemistry)
No of Positions:1
5.Department: Engineering Process Electrical
Area Wise Experience: Responsible for operation, Preventive Maintenance, Break down maintenance of process Equipment’s of Soft gelatin & Oncology Facility, Willingness to work in Shifts,
Designation: Ju. Executive / Executive
Experience: 2-5 years
Qualification: B. Tech/Diploma(Electrical)
No of Positions: 4
6.Department: Engineering Documentation(QMS)
Area wise Experience: Manage and update QMS documentation for engineering, ensuring integration into daily operations and compliance with internal/external standards.Oversee document control, versioning, traceability, and archiving in line with QMS requirements.Develop and maintain key planning documents like Calibration Planners, Criticality Assessments, PM Planners, and VMPs.Support engineering projects by preparing conceptual drawings and developing designs aligned with regulatory and operational standards
Designation: sr. Executive
Experience: 6-8 years
Qualification: B. tech
No of vacancies: 1
How to Apply?
Interview Location: Plot No: 28, Biotech Park Phase-III Karkapatla Village, Markook
Mandal, Siddipet Dist, Telangana- 502281
Date & Time: 06-06-2025 (Friday) & 10:00 AM to 01:00 PM
Please Carry the following Documents :
Latest Resume
Latest CTC Break-Up Annexure/Statement.
Photo Copies of Education Certificates, Aadhar & Pan Card Pay Slips-3 Months & One Passport Photo
In case of any query please connect hr@ciropharmagroup.com
Note: Candidates who are able to join immediately or within 30 days will be given preference.