About Company:
Gland Pharma is Established in Hyderabad, India in 1978, Gland Pharma is one of the fastest-growing small-molecule generic injectables-focused company, with a global footprint across 60 countries, including the United States, Europe, Canada, Australia, India and other markets in the rest of the world. Gland Pharma has seven manufacturing facilities in India, comprising four facilities for finished formulations and three API facilities. We are focused on meeting diverse injectables needs with a stable supply of affordable and high-quality products. We have established a portfolio of injectables products across various therapeutic segments and delivery systems and are present in sterile injectables, oncology and ophthalmics with a focus on complex injectables.
Website
http://www.glandpharma.com
Vacancy Details:
Job Description:
We’re hiring
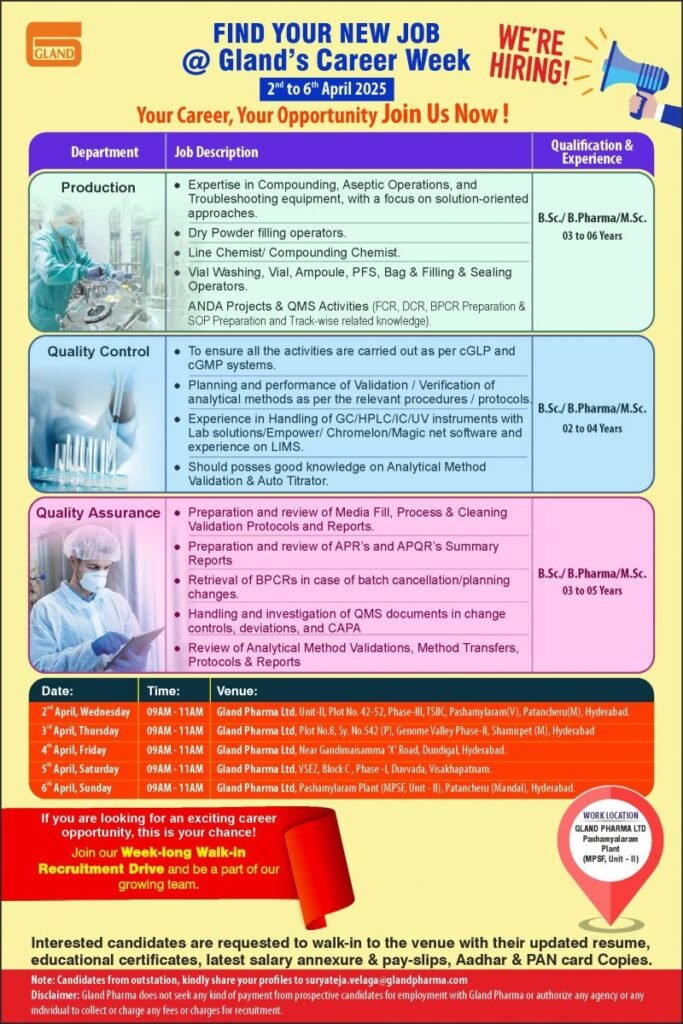
Gland’s Career Week || 2nd to 6th April, 2025
Looking for talented professionals who are excited about innovation, growth, and making a real impact.
Gland Pharma Ltd is hiring for Production, Quality Control & Quality Assurance Experienced Professionals to help us push boundaries and drive change.
Job Location: Unit 2, Pashamylaram, Telangana
Job Type: Full-time
Why Join Us?
1.Work with industry experts in a growing pharmaceutical company
2.Career growth opportunities and professional development
3.Competitive salary and benefits
1.Department: Production
Job Description:
1.Expertise in Compounding, Aseptic Operations, and Troubleshooting equipment, with a focus on solution-oriented approaches.
2.Dry Powder filling operators.
3.Line Chemist/ Compounding Chemist
4.Vial Washing, Vial, Ampoule, PFS, Bag & Filling & Sealing Operators.
5.ANDA Projects & QMS Activities (FCR, DCR, BPCR Preparation & SOP Preparation and Track-wise related knowledge).
Qualification & Experience: B.Sc./B. Pharma/M.Sc. & 03 to 06 Years
2. Department: Quality Control
Job Description:
1.To ensure all the activities are carried out as per cGLP and cGMP systems.
2.Planning and performance of Validation / Verification of analytical methods as per the relevant procedures / protocols.
3.Experience in Handling of GC/HPLC/IC/UV instruments with Lab solutions/Empower/ Chromelon/Magic net software and experience on LIMS.
4. Should posses good knowledge on Analytical Method Validation & Auto Titrator.
Qualification & Experience: 02 to 04 Years
3.Department: Quality Assurance
Job Description:
1.Preparation and review of Media Fill, Process & Cleaning Validation Protocols and Reports.
2.Preparation and review of APR’s and APQR’s Summary Reports
3.Retrieval of BPCRs in case of batch cancellation/planning changes.
4.Handling and investigation of QMS documents in change controls, deviations, and CAPA
5.Review of Analytical Method Validations, Method Transfers, Protocols & Reports
Qualification & Experience: 03 to 05 Years
Interested candidates are requested to walk-in to the venue with their updated resume, educational certificates, latest salary annexure & pay-slips, Aadhar & PAN card Copies.
Note: Candidates from outstation, kindly share your profiles to suryateja.velaga@glandpharma.com
Disclaimer: Gland Pharma does not seek any kind of payment from prospective candidates for employment with Gland Pharma or authorize any agency or any individual to collect or charge any fees or charges for recruitment.
For More Details Please Fallow the Below Image