About Company:
A consumer-led global pharmaceutical company, creating healthy doses of life since 1949. When you operate in an industry like pharmaceuticals, your work goes way beyond creating ‘products for customers’. It is different from any other domain – there lies a higher sense of responsibility and a need for utmost integrity in everything you do. As you serve millions of lives, high quality standards become a pre-requisite, and safety of your people and consumers always comes first. All this, while ensuring that each life you touch is treated with respect and dignity. For more than 60 years, Ipca has been a crucial healthcare partner in over 120 countries across the 6 continents. We are a fully-integrated pharmaceutical company that manufactures over 350 formulations and 80 APIs for various therapeutic segments. Today, we are one of the world’s largest manufacturers and suppliers of over a dozen APIs. These are produced from scratch at fully-automated manufacturing facilities, approved by the world’s most discerning drug regulatory authorities like UK-MHRA, EDQM-Europe, and WHO-Geneva, among others. – One of the largest suppliers of these APIs worldwide with manufacturing leadership in over 12 APIs globally – 15 APIs & 11 Formulations manufacturing facility across the globe Leader in DMARDs (Disease Modifying Anti-Rheumatic Drugs) treatment for Rheumatoid Arthritis – Leading brands in Pain, Rheumatology, Antimalarials and Hair care therapy – 4 formulations rank amongst the top 300 brands of IPM as per IQVIA
Website
https://www.ipca.com/
Vacancy Details:
Job Description:
Hiring Alert
Are you ready to upgrade your career
We’re on the hunt for someone who’s is passionate and thrive in a fast-paced environment. Apply now.
Perks and Benefit:
1.Transportation
2.Accommodation for bachelor
3.Training and learning material to improve skills.
4.Leave facilities.
Mention your Current CTC, Expected CTC and Notice period.
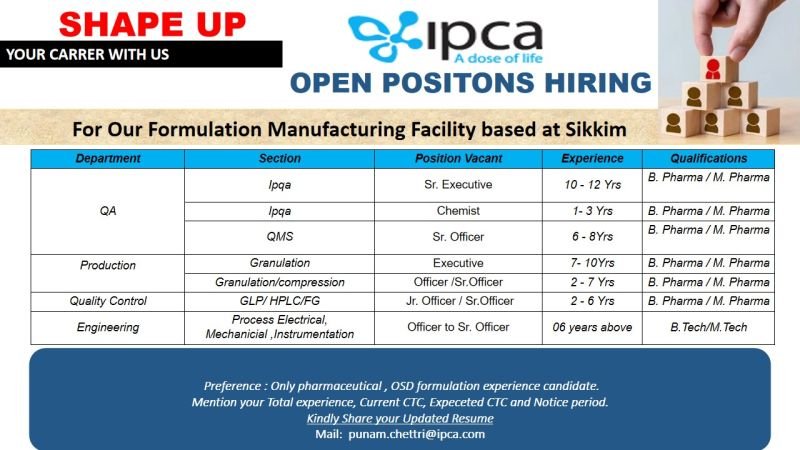
1.Dpartment: QA
a)Section: IPQA
Position Vacant: Sr. Executive
Experience: 10 to 12 years
Qualification: B. Pharma/M. Pharma
b). Section: IPQA
Position Vacant: Chemist
Experience: 1-3 years
Qualification: B. Pharma/M. Pharma
c). Section: QMS
Position vacant: Sr. Officer
Experience: 6-8 years
Qualification: B. pharma/M. Pharma
2. Department: Production
Section , Position & Experience: Granulation/Executive-7-10 years
Granulation/Compression- Officer/Sr. Officer 2-7 years
Qualifications: B. Pharma/M. Pharma
3.Department: Quality Control
Section: GLP/HPLC/FG
Position: Jr. Officer/Sr. Officer
Experience: 2-6 years
Qualifications: B. Pharma/M. Pharma
4.Department: Engineering
Section: Process Electrical, Mechanical, Instrumentation
Position: Officer to Sr. Officer
Experience: 6 years above
Qualifications: B. Tech/M. Tech