About Company:
Ajanta Pharma Limited (APL) is a pharmaceutical company headquartered in Mumbai, India. It has strong presence in Branded Generic business in India & Emerging markets; and Generic business in USA. In India, company operates in selected therapeutic areas of Cardiology, Dermatology, Ophthalmology and Pain management. Its brands hold leadership positions in sub-therapeutic areas they are present in. In Emerging Markets, company has presence in Africa, South East Asia, West Asia, and CIS on broader therapeutic segments such as antimalarial, gastro, antibiotics, cardiology, dermatology, pain management, etc. As on 30th Sept 2024, Ajanta has 46 ANDA approvals which are commercialised. It holds 2 tentative approvals and 22 ANDAs are awaiting US FDA approval. Company plans to file 8-12 ANDAs during the current financial year. Company has state-of-the-art research facilities for formulation and API development located at Mumbai, India. R&D capabilities are evident from number of 1st to market products launched by the company providing patients most needed compliance and convenience. A dedicated and focused team of over 800 scientists work for R&D, which is growing continuously. Ajanta has 6 formulations manufacturing facilities located in India. Besides that, it also has an API manufacturing facility located at Waluj, India. Ajanta’s flagship formulation facilities at Paithan (Maharashtra, India) and Dahej (Gujarat, India) have been approved by US FDA. Ajanta continuously invests in enhancing the existing manufacturing facilities to meet current cGMP requirements and also construct new facilities to meet company’s growth requirements. Please visit https://ajantapharma.com/ for more information.
Website
https://ajantapharma.com/
Vacancy Details:
Job Description:
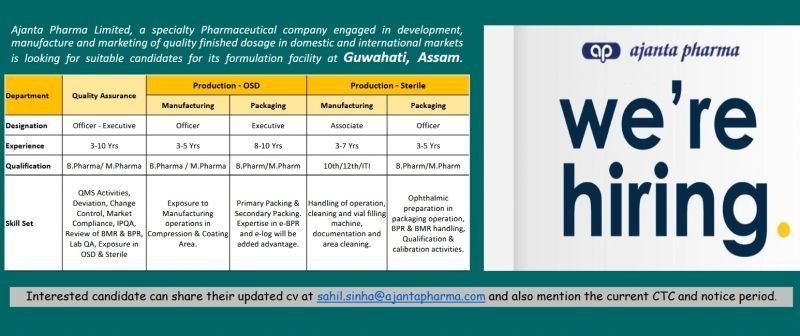
Ajanta Pharma Limited, a specialty Pharmaceutical company engaged in development, manufacture and marketing of quality finished dosage in domestic and international markets is looking for suitable candidates for its formulation facility at Guwahati, Assam.
1.Department: Quality Assurance
Designation: Officer – Executive
Experience: 3-10 Yrs
Qualification: B. Pharma/M. Pharma
Skill Set: QMS Activities, Deviation, Change Control, Market Compliance, IPQA Review of BMR & BPR, Lab QA, Exposure in OSD & Sterile
2.Department: Production-OSD(Manufacturing)
Designation: Officer
Experience: 3-5 Yrs
Qualification: B. Pharma/ M. Pharma
Skill Set: Exposure to Manufacturing operations in Compression & Coating Area
3.Department: Production-OSD(Packing)
Designation: Executive
Experience: 8-10 years
Qualification: B. Pharm/M. Pharm
Skill Set: Primary Packing & Secondary Packing. Expertise in e-BPR and e-log will be added advantage.
4.Department: Production-Sterile(Manufacturing)
Designation: Associate
Experience: 3-7 years
Qualification: 10th/12th/ITI
Skill Set: Handling of operation, cleaning and vial filling machine, documentation and area cleaning.
5.Department: Production-Sterile(Packing)
Designation: Officer
Experience:3-5 years
Qualification: B. Pharma/M. Pharma
Skill Set: Ophthalmic preparation in packaging operation, BPR & BMR handling. Qualification & calibration activities.
Area.
Email:
sahil.sinha@ajantapharma.com